- Main Page
- A1C Test
- Advance Directives
- Age on other Planets
- Aliens
- American Flag
- Angels
- Annuals
- Anxiety
- Aortic Aneurysm
- Apple Cider Vinegar
- Aphrodisiacs
- Arrhythmia
- Atrial Fibrillation
- Auras
- Avoiding Scams
- Awareness Ribbons
- Baileys Liqueur
- Bananas
- Banana Tree, Grand Nain
- Banana Tree, Ice Cream
- Banana Tree, Zebrina Rojo
- Beekeeping
- Benign P P Vertigo
- Birth Month
- Blood Tests
- Blood Test Tubes
- Blood Types
- Body Mass Index - BMI
- BMI Calculator
- Boogaloo
- Bookmarks
- Book of Shadows
- Book List
- Boot Anatomy
- Boot Fit Guide - Cowboy
- Boot Glossary
- Boot Leathers
- Boot Makers
- Boot Retailers
- Boot Styles - Western
- Boot Toes & Heels - Western
- Boot Toes & Heels - Work
- Bronchitis
- Candle Colors
- Carbohydrates
- Cardiac Catheterization
- Cardiovascular Disease
- Cats
- CGM's
- Chakras
- Chillicothe Businesses
- Chinese Zodiac
- Cholesterol
- Christmas Tree
- Ciroc Vodka
- Citalopram
- Coffee Pods
- Color Codes Chart
- Consumer Resources
- C.O.P.D.
- Coronary Artery Disease
- Country Stars
- Cowboy Hats
- Cowboy Hat Etiquette
- Cowboy Hat Sizing
- Cowboy Sites
- C.P.A.P.
- Craft Name!
- Credit Score Checkers
- Credit Scores
- Crystals & Gems
- CT scan
- Degenerative Disc Disease
- Deities
- Depression
- Diabetes Info.
- Diabetes Facts
- Diabetes - Pre
- Diabetes - Type 1
- Diabetes - Type 2
- Diabetes - Type 3c
- Diabetes - Gestational
- Diabetes Care
- Diabetes Care Team
- Diabetes Terms
- Diabetes Treatment
- Diabetes & Fruits
- Diabetes & Veg's
- Diet - Boiled Egg
- Diet - DASH
- Diet - Fat Burning
- Diet - Mediterranean
- Diet - Military
- Disability
- Disability Permits
- Disaronno Amaretto
- Do Not Resuscitate
- Donation
- Dream Catchers
- Dreams
- Drug Test
- Dupixent®
- Echocardiogram
- Electrocardiogram
- Electromyography
- Elementals
- Elements
- Emphysema
- Epsom Salt
- Eye Teasers
- Facet Arthropathy
- Fairies
- Fairies of Folklore
- Farxiga®
- Flower Astrology
- Fonts
- Foods To Regrow
- Fortune Teller
- Free For All Links
- Friend
- Funny Things
- Fun Stuff
- Gabapentin
- GERD
- Giving
- Glycemic Index
- Gout
- Growing Blueberries
- Halloween
- Halloween Treats
- Headaches
- Healing & Energy Work
- Health Facts
- Health Info. Lines
- Heart Attack
- Heart Disease - Other
- Heart Failure
- Heart Imaging Tests
- Hello!!
- Herbal Codes
- Herbal Medicine
- Herb & Oils Uses
- Herniated disc
- HIPAA
- Home Bar
- Home Remedies
- Horse Sites
- House Plants
- Humalog®
- Hunger Facts
- Hydrogen Peroxide
- Hyperglycemia
- Hypoglycemia
- Hyperkalemia
- Hypokalemia
- Hyperlipidemia
- Hypertension
- Hypotension
- Important Numbers
- Informed Consent
- Inhalers
- Insomnia
- Insulin
- Interesting Facts
- Juice Fasting
- Juice Recipes
- Karma
- Kidney Cysts
- Kidney Disease
- Kinds of Tea
- Lantus®
- Lemon Benefits
- Lime Benefits
- Logger vs Lineman
- Lucky Bamboo
- Lumbar Retrolisthesis
- Macaroni!!
- Magic 8 Ball
- Magick
- Medicaid
- Medicare
- Men's Health
- Mental Health
- Mirror Messages
- Missouri
- Missouri Prisons
- MO HealthNet
- Moon Phases
- Mounjaro®
- MRI Scan
- Muses
- Myelography
- Mystical Unicorn
- Naproxen
- Nasal Polyps
- Natal Astrology Chart
- National Foundations
- Need a Spell?
- Never Forget
- New Page Soon
- Nuclear Medicine
- Nutrition - Adults
- Nutrition - Adults, Older
- Nutrition - Kids
- Obesity
- One Little Rose
- Orchid Growing
- Orchid Sources
- Otolaryngologist
- Ouija
- Pagan Humor
- Pagans vs.Wiccans
- Parking Spaces
- PayPal.Me
- Pentagram vs. Pentacle
- Perennials
- Peripheral Artery Disease
- PET/CT Scan
- PET Scan
- Phobias A-Z
- Plant Care
- Plant Zone Map
- Potassium
- Propagating Plants
- Prurigo Nodularis
- Psychic Dictionary
- Psychic Gifts
- Psychic Reader
- Psychic Scams
- Psychic Test
- Psychometry
- PVC's
- Quartz Crystals
- Quit Smoking
- Radiculopathy
- Ragtime Music
- Recipes I like
- Red Yeast Rice
- Reiki
- Roses
- Runes
- Sadie & Beethoven
- Salt & Sodium
- Salt Water Flush
- Sciatica
- Scrying
- Séance
- Service Animals
- Shape Shifters
- Sleep Apnea
- Sleep Disorders
- Sleep Studies
- Smile
- SPECT Scan
- Speed Test
- Spices You Need
- Spices I Have
- Spinal Stenosis
- Spirit Guides
- Statins
- Stents
- Steel Toe vs. Comp. Toe
- Stress Test - Exercise
- Stress Test - Nuclear
- Sugars - Sweeteners
- Superstitions
- Support Groups
- Symbols
- Talisman
- Tarot
- Tea Leaf Reading
- Telekinesis
- Tequila Rose
- Testosterone
- The Ten Commandments
- Tools of the Craft
- Top Alcohol
- Top Animated Movies
- Top Comedy Movies
- Top Expensive Movies
- Top Modern Westerns
- Top 100 Westerns
- Toyota Yaris 2008
- Toyota Yaris 2012
- Trazodone
- Tree, Calamondin Orange
- Tree, Meyer Lemon
- Tree, Persian Lime
- Tree Signs
- Ultrasound
- US Bill of Rights
- US Constitution
- US Declaration of Independence
- UV Vodka
- Vaccines by Age
- Vaccines 0-6 yrs
- Vaccines 7-18 yrs
- Vaccines 19 and up
- Ventricular Fibrillation
- Vertigo
- Vital Records
- Vital Signs
- Vitamin B12
- Vitamin C
- Vitamin D
- Vitamin E
- Vitamin F
- Vitamin K
- Vitamins & Minerals
- Vitamins Recommended
- Water Benefits
- Weight on other Planets
- Wheel of the Year
- White Magick
- Wicca
- Wiccan Rede
- Wine Clubs
- Wines
- Wines - Missouri
- X-Rays
- Yin / Yang
- Zodiac Signs
Needed to read PDF's
Mounjaro®

Pronunciation: mown-JAHR-OH
Generic name: tirzepatide
Dosage form: single-dose injection pen (multiple strengths), single-dose vial (multiple strengths)
Drug class: GLP-1 Agonists (Incretin Mimetics)
What is Mounjaro?
Mounjaro (tirzepatide) is used for type 2 diabetes to help lower blood sugar levels, and tirzepatide also causes weight loss. Mounjaro is a once-weekly injection that should be used alongside dietary changes and exercise.
Mounjaro received FDA approval on May 13, 2022. There is no Mounjaro generic. The 2 different brands of tirzepatide (Mounjaro and Zepbound) are made by Eli Lilly and Company but are FDA-approved for different conditions.
- Mounjaro: Specifically approved for type 2 diabetes management in adults.
- Zepbound: Designated for weight loss treatment in adults.
Benefits of Mounjaro?
- Blood sugar control and HbA1C reduction.*
- Significant weight loss support.**
*HbA1C measures your average blood sugar level over the past 2-3 months.
** Not an FDA-approved use.
How Does Mounjaro Work?
Mounjaro's mechanism of action involves mimicking a natural hormone in the body called GLP-1 which:
- Stimulates insulin production from the pancreas
- Reduces liver sugar production
- Slows down digestion
- Helps control appetite and food intake.
Mounjaro belongs to the drug class called GLP-1 receptor agonists.
Medical Uses and FDA Approval
Mounjaro is officially approved by the FDA for:
- Blood sugar (glucose) control in adults with type 2 diabetes, along with diet and exercise.
Although Mounjaro for weight loss is not an official FDA approval, Mounjaro has been shown to help weight loss and maintain the lost weight in clinical trials when combined with diet and exercise.
WARNING: Mounjaro is not approved for type 1 diabetes treatment and it is not known if it can be used in people with pancreatitis.
It is not known if Mounjaro is safe or effective in children under 18 years of age.
Mounjaro side effects
Common Mounjaro side effects
The most common Mounjaro side effects are nausea, diarrhea, decreased appetite, vomiting, constipation, heartburn (dyspepsia), and abdominal pain.
These gastrointestinal effects typically affect more than 5% of patients, usually improve within weeks, and are more frequent at higher doses. People are more likely to discontinue Mounjaro due to stomach side effects if they are on a higher Mounjaro dosage (5mg dose: 3.0% risk of stopping treatment vs 15mg dose: 6.6% risk)
Tips for managing Mounjaro stomach side effects
Following these tips may help you manage Mounjaro's stomach side effects:
- eat more slowly
- consume smaller meals
- select more bland, low-fat foods (like crackers, toast, and rice)
- avoid greasy, fried foods or sugar treats
- eat foods that contain water (like soup or gelatin)
- don’t lie down right after eating
- drink clear or ice-cold liquids
- if possible, go outside for fresh air if you feel sick.
Serious Mounjaro side effects
Mounjaro can cause serious side effects such as inflammation of the pancreas, vision changes, low blood sugar levels, kidney problems, and serious allergic reactions, and it can also increase the risk of food or liquid getting into your lungs during surgery or a medical procedure.
Get emergency medical help if you have signs of an allergic reaction, such as hives, itching, dizziness, fast heartbeats, difficulty breathing, or swelling of your face, lips, tongue, or throat.
Call your doctor at once if you have:
- vision changes;
- unusual mood changes, thoughts about hurting yourself;
- pounding heartbeats or fluttering in your chest;
- a light-headed feeling, like you might pass out;
- signs of a thyroid tumor - swelling or a lump in your neck, trouble swallowing, a hoarse voice, feeling short of breath;
- symptoms of pancreatitis - severe pain in your upper stomach spreading to your back, nausea with or without vomiting, fast heart rate;
- gallbladder problems - upper stomach pain, fever, clay-colored stools, jaundice (yellowing of the skin or eyes);
- low blood sugar--headache, hunger, weakness, sweating, confusion, irritability, dizziness, fast heart rate, or feeling jittery;
- kidney problems - swelling, urinating less, blood in urine, feeling tired or short of breath
- stomach flu symptoms - stomach cramps, vomiting, loss of appetite, diarrhea (may be watery or bloody)
- symptoms of ileus (stomach paralysis) - bloating, stomach cramps or pain, nausea or vomiting, constipation or diarrhea, loss of appetite.
Food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Mounjaro may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Mounjaro before you are scheduled to have surgery or other procedures.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Warnings
Possible thyroid tumors, including cancer. In clinical trials with rodents, Mounjaro and medicines that work like Mounjaro caused thyroid tumors, including thyroid cancer. It is not known if Mounjaro will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer.
- Do not use Mounjaro if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Keep all appointments with your doctor and the laboratory. Your doctor may order certain tests to check your body's response to this medicine.
Before using this medicine
You should not use this medicine if you:
- are allergic to the tirzepatide, Mounjaro, Zepbound, or any of the ingredients contained in the pens or vials (see below for a list of ingredients)
- have a personal or family history of medullary thyroid carcinoma (a type of thyroid cancer); or
- multiple endocrine neoplasia type 2 (tumors in your glands).
Tell your doctor if you have ever had:
- pancreas problems;
- kidney disease;
- are planning surgery or other procedure that uses anesthesia or deep sedation
- a severe stomach problem such as problems with digesting food or slowed emptying of your stomach (gastroparesis); or
- diabetic retinopathy (a diabetes complication that affects the eyes).
Pregnancy
Tell your healthcare provider if you are pregnant, you become pregnant, or plan to become pregnant while using this medicine. It is not known if this medicine will harm your unborn baby.
This medicine can make birth control pills less effective. Ask your doctor about other birth control options such as an injection, implant, skin patch, vaginal ring, condom, diaphragm, cervical cap, or contraceptive sponge. If you take birth control pills, you may need to use additional birth control methods for 4 weeks after starting this medicine, and for 4 weeks each time, the dose is raised.
Breastfeeding
Tell your healthcare provider if you are breastfeeding or plan to breastfeed while using this medicine. It is not known whether this tirzepatide passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using this medicine.
How should I use Mounjaro?
You should read the Instructions for Use carefully and ask your pharmacist or doctor to explain any part you do not understand. It is important to use this medicine exactly as directed. Do not take more or less of it or more often than your doctor prescribes.
Mounjaro is a pre-filled pen injected under the skin (subcutaneously).
- Mounjaro is usually given 1 time a week
- It can be given with or without meals at any time of the day
- It may be injected under the skin of the thigh, abdomen, or upper arm.
- You should rotate the injection site for each dose.
Your healthcare provider will usually start you on a low dose, which will be gradually increased, but not more than once every 4 weeks.
You may change the day of the week you use this medicine as long as there are at least three days between doses.
You may give insulin in the same area as this medicine, but they should not be given right next to each other. Do not mix insulin and Mounjaro in the same injection.
Mounjaro controls type 2 diabetes but does not cure it. It may take four weeks or longer before you see the full benefit of this medicine. Continue to take this medicine even if you feel well. Do not stop taking this medicine without talking to your doctor.
Mounjaro Dosing Information
Usual Adult Dose for Diabetes Type 2:
Initial dose: 2.5 mg under the skin (subcutaneously) once a week.
After 4 weeks: Increase to 5 mg subcutaneously once a week.
If additional glycemic control is needed: Increase from 5 mg to 7.5 mg for 4 weeks and thereafter in 2.5 mg increments after at least 4 weeks on the current dose.
Maximum dose: 15 mg subcutaneously once a week.
Comments: The 2.5 mg dosage is for starting treatment and is not intended for glycemic control. The day of weekly administration can be changed, if necessary, as long as the time between the 2 doses is at least 3 days (72 hours).
What strengths are Mounjaro pens?
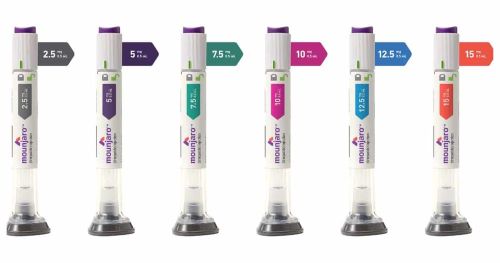
Mounjaro is available as a single-dose autoinjector pen or vial in the following strengths:
- 2.5 mg/0.5 mL (Grey)
- 5 mg/0.5 mL (Purple)
- 7.5 mg/0.5 mL (Green)
- 10 mg/0.5 mL (Pink)
- 12.5 mg/0.5 mL (Blue)
- 15 mg/0.5 mL (Orange)
In the past, there have been issues with Mounjaro availability due to increased demand leading to drug shortages for some strengths.
What should I do if I miss a dose?
Take the missed dose as soon as you remember it within 4 days after the missed dose.
However, if more than 4 days have passed, skip the missed dose and continue your regular dosing schedule. Do not inject two doses within 3 days of each other.
What should I do in case of an overdose?
In case of overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at Poison Help If the victim has collapsed, had a seizure, has trouble breathing, or can't be awakened, immediately call emergency services at 911.
What other drugs affect Mounjaro?
Tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
Birth control pills may not work as well while receiving Mounjaro. Your doctor may suggest you use another form of birth control for 4 weeks after starting this medicine and for 4 weeks after each dose change.
Taking other medicines to treat diabetes like insulin, sulfonylureas, or other GLP-1 agonists such as Ozempic (see Mounjaro vs Ozempic) with this treatment may increase your risk of low blood sugar levels. Talk to your doctor and pharmacist about low blood sugar and how to manage it.
This medicine delays gastric emptying and has the potential to change the absorption of other medicines that are taken orally.
This list is not complete. Many other drugs may interact with this medicine, including prescription and over-the-counter medicines, vitamins, and herbal products.
How do I store Mounjaro?
Store your Mounjaro pens in the refrigerator between 36°F to 46°F (2°C to 8°C) in their original carton to protect them from light.
If needed, or while traveling, each single-dose Mounjaro pen can be stored at room temperature up to 86F (30C) for up to 21 days.
Who makes Mounjaro?

Mounjaro is made by Eli Lilly and Company, commonly known as Lilly. They are an American pharmaceutical company based in Indianapolis that was founded in 1876.
Notable drugs from Lilly include:
- Zepbound (tirzepatide for weight loss - same molecule as Mounjaro)
- Trulicity (diabetes)
- Verzenio (breast cancer)
- Jardiance (diabetes, heart failure - partnered with Boehringer Ingelheim)
- Cymbalta (depression, anxiety)
- Prozac (depression - though now generic)
- Olumiant (rheumatoid arthritis)
- Kisunla (Alzheimer's - recently approved).
Find me on Social Media
 |
Don't forget to bookmark me to see updates.. Copyright © 2000 - 2025 K. Kerr Most recent revision June 11, 2025 10:39:00 PM |







